Role of cell shape dynamics as instructive cues directing tissue morphogenesis
Research in my lab addresses the fundamental question of how complex tissues arise by the process of morphogenesis – whereby progenitor cell populations elegantly reorganise, pattern and differentiate to form distinct organs and tissues. Tissue morphogenesis is associated with dramatic changes in cell shape that define final tissue form. Yet, far from simply sculpting tissue architecture, our research reveals that these cell shape dynamics also encode critical instructive cues that direct key morphogenetic events underpinning tissue-building. As such, our work aims to define the precise role and regulation of morphogenetic cues encoded in cell shape, as well as utilise this information to predictably direct tissue formation.
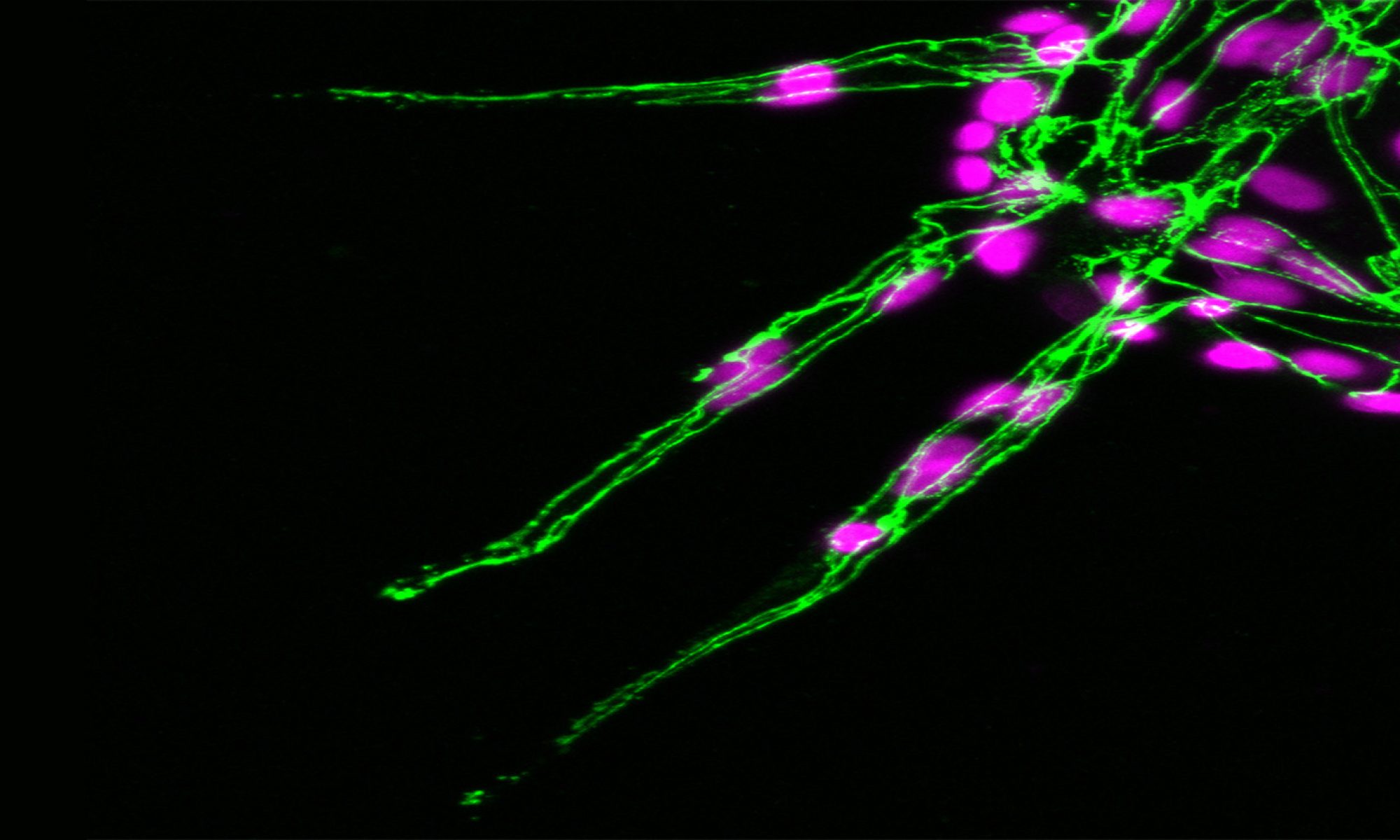
To this end, we exploit the vertebrate vasculature as a key morphogenetic model to define critical cues encoded in cell shape. Blood vessel formation (or angiogenesis) is a paradigm model for study of the core mechanisms underpinning morphogenesis, including broadly conserved morphogenetic events such as collective cell migration, asymmetric cell division, competitive cell fate decisions, branching morphogenesis, tissue patterning and tubulogenesis. Moreover, dysregulation of angiogenesis underpins numerous pathological processes, including tumour growth and metastasis, retinopathy and blindness, arthritis, limb ischemia and atherosclerosis. Hence, not only do studies of angiogenesis shed light on the fundamental principles of tissue-building, but novel insights also have clear therapeutic potential.
To explore how cell shape cues direct fundamental decision-making processes driving tissue formation, we adopt a highly interdisciplinary multiscale approach integrating: (1) in vivo and in vitro live-cell imaging in the zebrafish model system and human primary cells, (2) optogenetics, signalling reporters and other genetic transgenic tools, (3) in vitro micropatterning and manipulations of cell shape, (4) ‘Omics’ techniques, (5) CRIPSR gene editing, and (6) computational modelling.
Exploiting these approaches, in recent years we’ve revealed unexpected roles for cell shape dynamics in directing asymmetric cell divisions (Lovegrove et. al., 2025. Science; Costa et. al., 2016. Nat. Cell Biol.), organelle positioning (Bradbury et. al. 2025. bioRxiv), tissue guidance (Costa et. al. 2020. EMBO J.), and competitive cell fate decisions (Zakirov et. al. 2021. Philos. Trans. R. Soc. Lond. B). Considering that cell shape change is an inherent feature of almost all forming tissues, mechanisms we uncover are likely widely conserved and of broad therapeutic relevance in diverse tissue contexts.